DEVELOPMENT & VISION
JIRD is a pharmaceutical marketing company focused on developing a wide range of medical products, including solid and semi-solid dosage forms, as well as sterile injectables.
- Founded in 2003 by Dr. Ousama Khair in Jordan, JIRD expanded rapidly to Tunisia. Dr. Khair, the current CEO, brings over 25 years of experience in North African and Middle Eastern pharmaceutical markets.
- By 2009, JIRD expanded internationally under "AFMA International".
Additionally, it established "Natubio," a subsidiary specializing in cosmetics and food supplements. JIRD strategically integrates pharmaceutical facilities to expand its product portfolio and global market presence. Through investments in other facilities, JIRD ensures its products meet the highest industry standards.
GMP CERTIFICATION
Good manufacturing practices GMP is a concept of quality assurance, they are applied to pharmaceutical products for which they constitute a regulatory framework enforceable during inspections of pharmaceutical establishments by their supervisory authorities
QUALITY CONTROL
The development procedures for food supplements are the same applied to pharmaceutical products, and are subject to strict manufacturing procedures (adapted analytical dosages: Gas Chromatography - Mass Spectrometry (GC-MS) coupling, HPLC tests, impurities, stability studies , microbiological analysis...) Rigorous internal quality control (internal audit) equivalent to pharmaceutical products, from raw material to finished product.
Ambition
We combine honesty, ambition and a sense of responsibility. We give our employees the opportunity to develop their potential.
Solidarity
Solidarity is the ointment that creates communion and facilitates and enhances cohesion between our colleagues. In a climate of mutual assistance, all members of our team manage to flourish and evolve normally, which generates the development of our company in a harmonious framework.
Services
Check our partners and Services
bioequivalence studies Guarantee of effectiveness and safety
Carrying out our bioequivalence studies according to international Guidelines and in reference centers, compliant and approved by the European Union: PRU center, Biocenter, Om sai clinical research Pvt.
Certified in compliance with the quality requirements of the European Pharmacopoeia
According to the European Directorate for the Quality of Medicines (EDQM).
Certified Raw Material Purity
The purity of raw materials is approved by purity certificates compliant and updated with national (LNCM) and international (EMA) standards.
The experience of years
The years of our medicines and the trust of our doctors and patients are proof of pride in our COMMITMENT TO QUALITY.
Have confidence and inspire confidence.
Creating a climate of mutual trust requires above all the practice of transversal communication by encouraging exchanges and relying on the skills, talents, and potential of each member of our team. By opting for management through trust, we ensure continuous motivation and a constant sense of responsibility among our employees.
Discipline
Within our company, discipline is an essential component to the proper functioning of our organization in order to preserve our brand image and achieve our objectives.
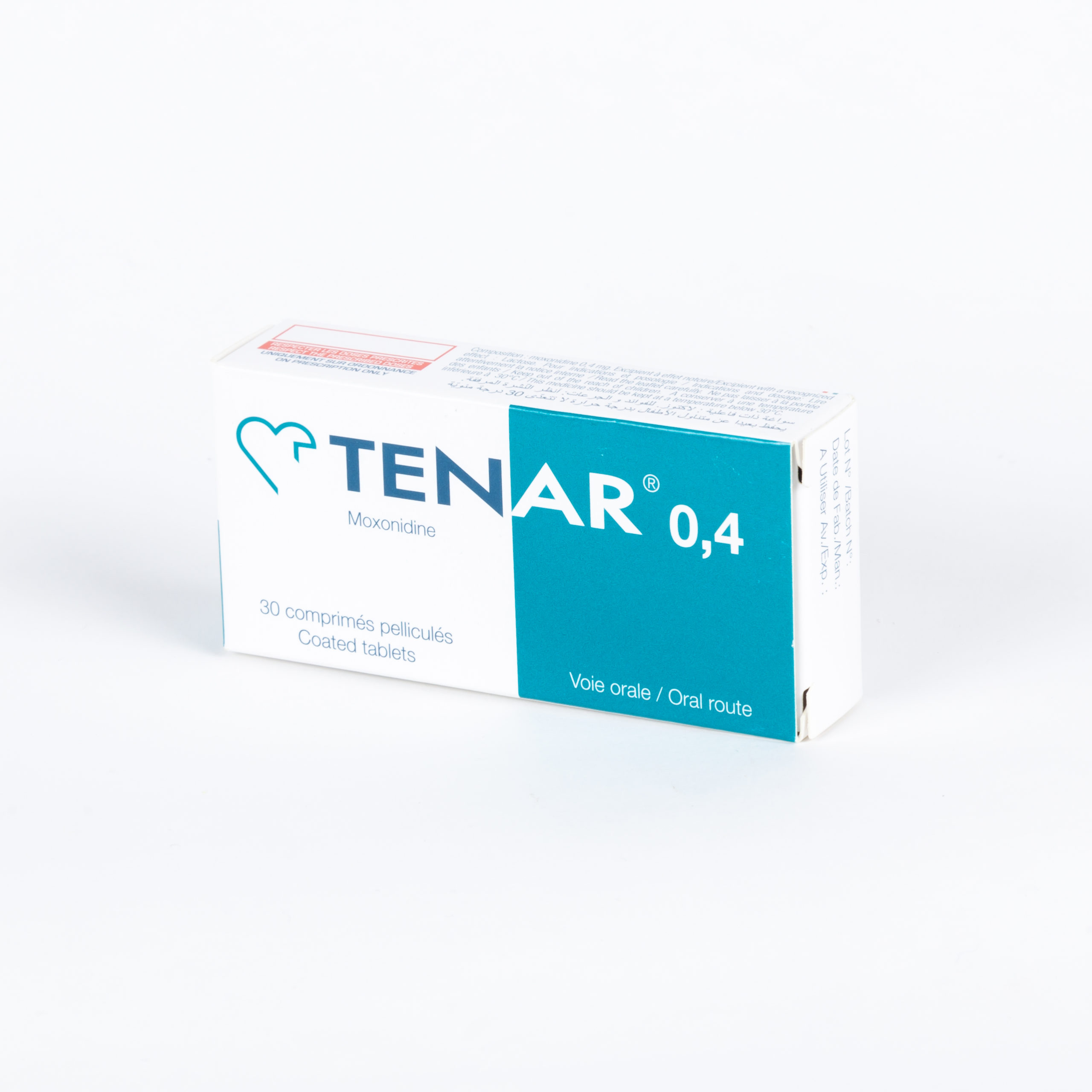
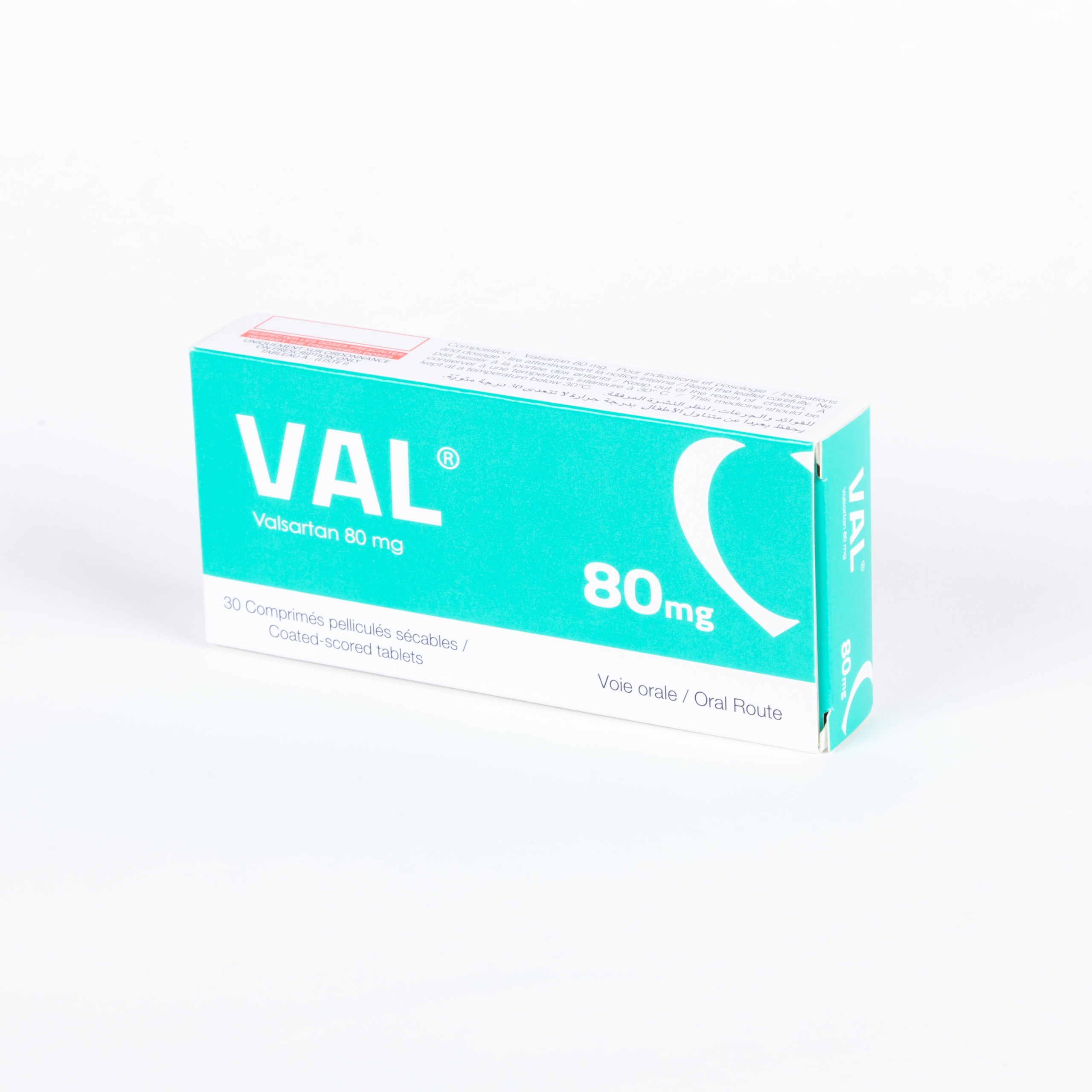
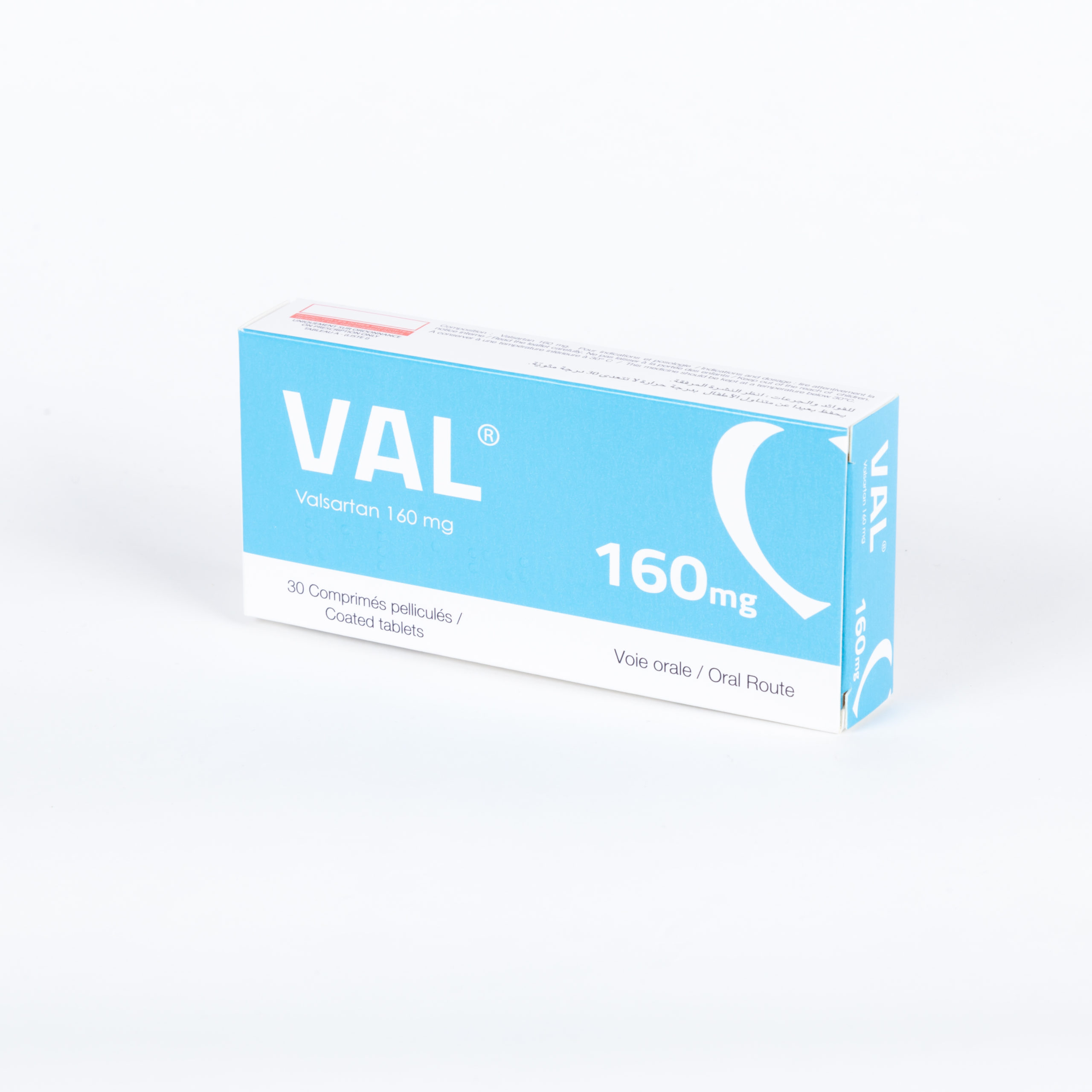
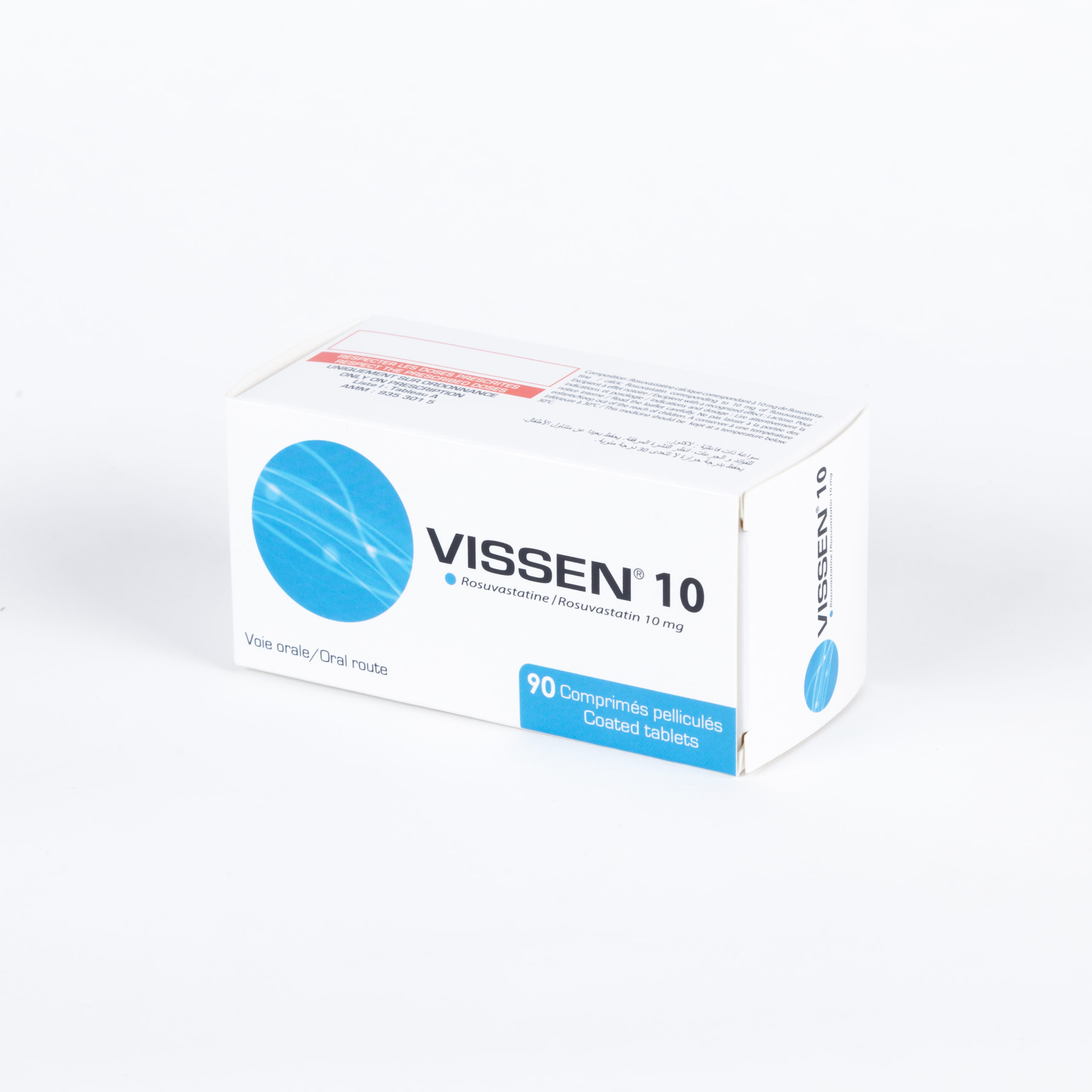
Portfolio
Check our Portfolio
- All
- Cardiology
- Neuropsychiatry
- Food supplement
- Pain Relief
- Dermatology
- Gynecology
- Gastrology
Key Highlights of Our Commitment
Witness our dedication to elevating product standards through meticulous quality control, innovative approaches developed over 25 years, specialized offerings with a focus on wellness, and a skilled team comprising pharmacists and doctors.
Quality PRODUCTS compliance with good manufacturing practices
YEARS experience and innovation
SPECIALITES with selgon Health priority
EMPLOYES Pharmacists and doctors
Contact
Contact Us
Location:
Al Sawalmeh Center, Al Dammam St 24, Amman, Jordan
Email:
infomed@philadelphiapharma.net
Call:
(+216)29 945 991